Fertilization requires extracellular Calcium
Explainer for our 2017 publication in PLoS One:
The requirement for extracellular Ca2+ for fertilization in the African clawed frog, Xenopus laevis, was controversial. For starters, it was widely accepted amongst sperm physiologists that Ca2+ entry into the male gamete is required for the acrosome reaction in sperm from nearly all species, including X. laevis. However, a 1998 study conducted by Wilkinson et al, demonstrated that eggs inseminated in the presence of 5 mM of the Ca2+ chelator BAPTA, fertilized and initiated normal embryonic development. BAPTA has a very high affinity for Ca2+, with a Kd of 110 nM. Coupled with the knowledge that the Wilkinson study used standard fertilization solutions to conduct their experiments (containing less that 1 mM of added CaCl2), there shouldn’t be any Ca2+ around to enter sperm and signal the acrosome reaction in the presence of 5 mM BAPTA. The reported ability of sperm to fertilize eggs in the presence of 5 mM BAPTA piqued our interest. So, we sought to uncover whether extracellular Ca2+ is required for X. laevis fertilization. If extracellular Ca2+ is required, when is it needed?
To start these experiments, we simply asked whether X. laevis fertilization and early embryonic development occurs in the absence of added CaCl2. For these experiments, we used the appearance of cleavage furrows as an indicator of embryonic development:
Shown are an egg (left) and an 8-cell embryo (right).
We found that X. laevis eggs inseminated in a solution lacking added divalent cations (no CaCl2 or MgCl2), developed normally:
Added Ca2+ is not required for the development of embryonic cleavage furrows
These results were surprising in the context of the requirement of Ca2+ entry for the acrosome reaction, but were consistent with the Wikinson manuscript. So, we continued on with our experimentation by experimentally determining whether early embryonic development progressed normally in the presence of varying BAPTA concentrations:
Incidence of cleavage furrow development decreased, with increasing concentrations of BAPTA.
We found fewer cleavage furrows in eggs inseminated in higher concentrations of BAPTA. Fitting these data with a sigmoidal function, we calculated an averaged half-maximal inhibitory concentration (IC50) of 519 ± 76 μM. This result was surprising to us for a two reasons: First, we were finding that BAPTA was preventing early embryonic development – the exact opposite of the findings reported in the Wilkinson manuscript; Second, our detected IC50 (519 μM) was MUCH higher than the Kd of BAPTA for Ca2+. Due to this latter finding, we were worried that BAPTA was interfering embryonic development by non-specific actions, independent of Ca2+ chelation. Thus, we tested for possible non-specific effects in two ways. First, we chelated Ca2+ with a different compound, EGTA:
Incidence of cleavage furrow development decreases when eggs are inseminated in increasing concentrations of EGTA.
Again, we found fewer cleavage furrows in eggs inseminated in higher EGTA concentrations, with an averaged IC50 of 178 ± 25 μM (to interrupt this story for a moment: you may have heard that BAPTA is the best Ca2+ chelator available and are wondering how EGTA is seemingly more effective for these experiments – exerting the half maximal effect at a lower concentration. The answer is that EGTA binds to Ca2+ with a higher affinity than BAPTA does, with a Kd of about 10 nM at pH 7.8. The reason why BAPTA is better than EGTA, is simply because it’s Ca2+ affinity is not pH dependent. BAPTA was based on EGTA – and both originally synthesized by Roger Tsien and colleagues, based on EDTA…which binds both Ca2+ and Mg2+ with a relatively high affinity, making it unsuitable for our experiments. Also, a common misconception is that each BAPTA molecule binds two Ca2+ ions; in the original BAPTA manuscript, Roger Tsien convincingly demonstrates with NMR that each BAPTA molecule binds to only ONE Ca2+ ion).
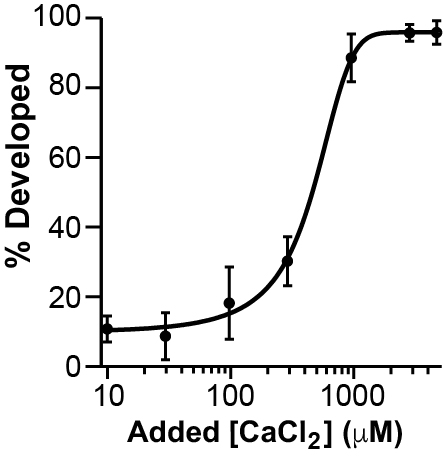
As a second approach, we determined whether we could recover the BAPTA-induced disruption of early embryonic development, by inseminating in BAPTA and saturating Ca2+ concentrations. For these experiments, we inseminated in 1 mM BAPTA (a concentration that completely prevented embryonic development in our own hands), and varying Ca2+ concentrations ranging from 10 μM to 5 mM.
Adding CaCl2 restores the appearance of cleavage furrows in eggs inseminated in 1 mM BAPTA
We again used the sigmoidal function to fit these data, yielding a half-maximal effective Ca2+ concentration (EC50) of 420 ± 40 μM. Together these data demonstrate that extracellular Ca2+ rescues the BAPTA disruption of early embryonic development. With all of our EGTA data collected thus far, we felt confident that Ca2+ chelators indeed disrupt embryonic development in X. laevis.
We next turned our attention to when in early embryonic development extracellular Ca2+ may be required. Many important physiologic events occur in the 90 minutes that pass between sperm application and the appearance of cleavage furrows. Honing-in on a time frame would illuminate when extracellular Ca2+ may be required. To determine when embryonic development requires Ca2+, we conducted transfer assays. For these experiments, eggs were inseminated with or without 3 mM BAPTA. After incubating the gametes together for 30 minutes, the inseminated eggs were washed twice and transferred to a different solution: with or without 3 mM BAPTA:
We found that none of the eggs inseminated in 3 mM BAPTA developed cleavage furrows, regardless of whether they were transferred to a solution with or without BAPTA. These results suggested that BAPTA is disrupting events occurring within the first 30 minutes of insemination.
Eggs inseminated in the presence of 3 mM BAPTA or higher failed to develop cleavage furrows in all experimental conditions examined thus far. However, these assays have not distinguished between inhibition of events occurring before or immediately after fertilization.
The experimental data that we had collected thus far demonstrated that extracellular Ca2+ is required near the time of fertilization. None of the assays performed until now distinguished between inhibition of events occurring before or immediately after fertilization. One fertilization event thought to require extracellular Ca2+ is the acrosome reaction, and 50 mM EGTA blocks the in vitro induction of it in X. laevis sperm. In the acrosome reaction, sperm release enzymes into the extracellular space that facilitate penetration of the matrix surrounding the egg. In X. laevis, this matrix is called the vitelline envelope. We reasoned that if BAPTA was preventing sperm from undergoing the acrosome reaction, then sperm would only penetrate the vitelline envelope of eggs inseminated without the Ca2+ chelator. To test this experimentally, we labeled sperm with Hoescht and using fluorescence imaging, we assayed for their presence in the perivitelline space.
We found several sperm in the perivitelline space of eggs inseminated without BAPTA, but no sperm in the perivitelline space of eggs inseminated with BAPTA. These data demonstrate that sperm do not penetrate the vitelline envelope in the presence of BAPTA and are consistent with the hypothesis that BAPTA prevents fertilization by preventing sperm from undergoing the acrosome reaction.
At this point, we wondered how Wilkinson et al. documented embryonic development of eggs inseminated in the presence of BAPTA. Going back to the 1998 manuscript, the authors indicated that eggs were inseminated in 5 mM BAPTA, and 5 minutes after sperm addition, the eggs were transferred to a Ca2+-containing solution. We hypothesized that sperm can penetrate the jelly layer surrounding the eggs in the presence of BAPTA, but wait to undergo the acrosome reaction until extracellular Ca2+ is restored. Only sperm within the jelly coat would be transferred to a new solution. We tested this hypothesis by inseminating eggs under various conditions, washing the embryos, and then transferring the eggs to new conditions 5 minutes after sperm addition. We found that embryos inseminated in BAPTA but transferred to a Ca2+ containing solution indeed developed cleavage furrows. Further supporting our hypothesis that sperm penetrate the jelly BAPTA but do not fertilize, we found that the first cleavage furrows appeared approximately 10 minutes later for these embryos compared to embryos inseminated in and transferred to control solutions.
At this point, it was not yet clear how solutions with no added Ca2+ (and should have low Ca2+ concentrations, on the order of tens of nM), could support fertilization and early embryonic development at yet milimolar concentrations of the Ca2+ chelators were required to interfere with embryonic development. We turned to the jelly layer surround the egg and wondering whether it may serve as a buffer for freely available Ca2+, to support fertilization. We then estimated the diffusible Ca2+ content from the jelly using fura-2 photometry. For these experiments, we incubated X. laevis eggs in solutions with no Ca2+ added, collected these solutions, and measured the Ca2+ that had diffused from the jelly layer, into the surrounding solutions. In four trials, an average of 17.5 ± 4.7 nmol of Ca2+ diffused from each jellied egg into the surrounding. We found that jellied eggs have an averaged diameter of 2.0 ± 0.2 mm, and 1.4 ± 0.02 mm for the egg alone. Based on these measurements and the assumption that the egg and surrounding jelly coat are spherical, we estimate that the averaged jelly volume to be 2.8 μl (N = 11 jellied eggs from 4 frogs). Accordingly, we estimate that the averaged concentration of freely-diffusing Ca2+ in the jelly coat is 6.3 ± 1.7 mM.
In summary, we have found that extracellular Ca2+ is required for X. laevis fertilization and that the jelly layer surrounding the egg is enriched with freely diffusing Ca2+ ions. Our data suggest that BAPTA and EGTA interfere with the development of cleavage furrows by preventing sperm from undergoing the acrosome reaction and speculate that the jelly layer is enriched in Ca2+ to support fertilization under changing environmental conditions.
This work was performed by everyone in the Carlson lab at the time of publication!